Here are some examples of single replacement reactions. A chemical reaction in which an element replaces one element in a compound.

Single Replacement Reaction Definition And Examples
This can either be in the form of a single replacement reaction or a double replacement reaction.
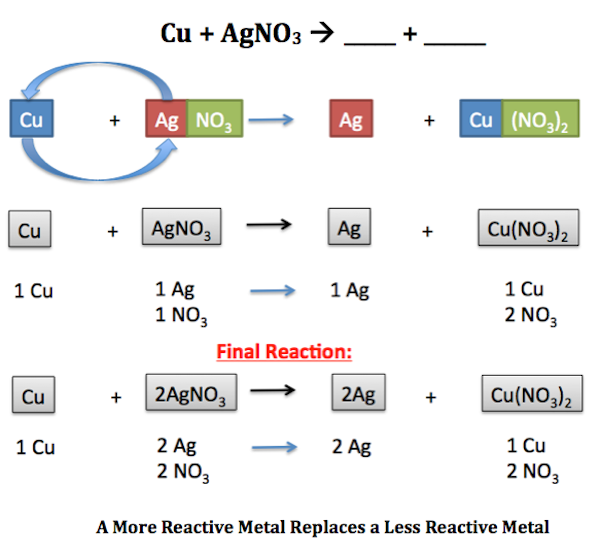
. In this equation A represents a more reactive element and BC represents the original compound. A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. A BC B AC.
X Y XYAs it is possible to warn X and AND are two different chemicals that combine to form a new product XY. - A BC AC B - Example. A double-replacement reaction exchanges the cations or the anions of two ionic compounds.
A single-displacement reaction also known as a single-replacement reaction is a type of chemical reaction where an element reacts with a compound and takes the place of another element in that. A single amount of insurance applying to more than 1 building andor contents. Definition Single Replacement is a reaction in which an element and compound or solution yield a new element and new compound or solution The reactants are an element and compound and the products are a different element and different compound.
A single displacement reaction which is also called as single replacement reaction is a kind of oxidation-reduction chemical reaction when an ion or element moves out of a compound ie one element is replaced by the other in a compound. A single replacement reaction also known as a single displacement reaction occurs when one element in a molecule is swapped for another. In single replacement reactions one ion or an atom of an element is displaced by another ion or atom discussed in detail below.
A single-displacementreaction also known as a single-replacementreaction is a type of chemical reaction where an element reacts with a compound and takes the place of another element in that. Looking for single-replacement reaction. A single-displacement reaction is a chemical reaction where one reactant is exchanged for one ion of a second reactant.
Involves metals and ionic compounds. During the reaction A replaces B forming the product compound AC and releasing the less reactive element B. A single replacement reaction aka single displacement reaction will occur if M 1 cation is less reactive than M 2The reactivity order corresponds to the reactivity series of the metals.
Generic Word Equation Element Compound yields New element New compound Must use the Activity. A wall that is not part of the structural support of a building and is intended through its design and construction to collapse under specific lateral. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions.
Ca H2O CaOH2 H2. In a single replacement reaction one of the reactants is more reactive than the other which results in the formation of a product that is more stable. ZnCuCl2 Cu ZnCl2.
The historical figure who is considered to be the first to give real and conscious form to the synthesis reactions is the German chemist Friedrich Wholer 1800 1882 who achieved this result from. ABC B AC. A replacement reaction is a type of chemical reaction in which one element replaces another in a compound.
2 K 2 H 2 O 2 K O H H. Neutralization precipitation and gas formation are types of double. The formula of a synthesis reaction is as follows.
When components of two ionic compounds are swapped two new compounds are formed. In spite of three hip replacements Adams manages to visit his local pub to play. It is also known as a single-replacement reaction.
Thats known as a single replacement chemical reaction and it results in a brand new product. The periodic table or an activity series can help predict whether single-replacement reactions occur. 1 The action or process of replacing someone or something.
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. An example of a single replacement reaction occurs when potassium K reacts with water H 2 O. Metal displaces metals while non-metals displace non-metals.
Find out information about single-replacement reaction. Blanket insurance is not permitted under the NFIP. A metal replaces another metal that is in solution.
Metals are usually in solutions. Examples of Single Replacement Chemical Reactions. Displacement reactions can be further classified into single replacement reaction and double replacement reaction.
Also known as a single replacement. Sometimes a chemical reaction takes an element away from a compound and adds it to another substance A BC B and AC. In a double replacement reaction there is interchanging of ions or atoms between the reactants.
A single-replacement reaction replaces one element for another in a compound. 1 element or compound replaces another element or compound in a compound. Single displacement reactions take the form.
There are two types of single replacement reactions.
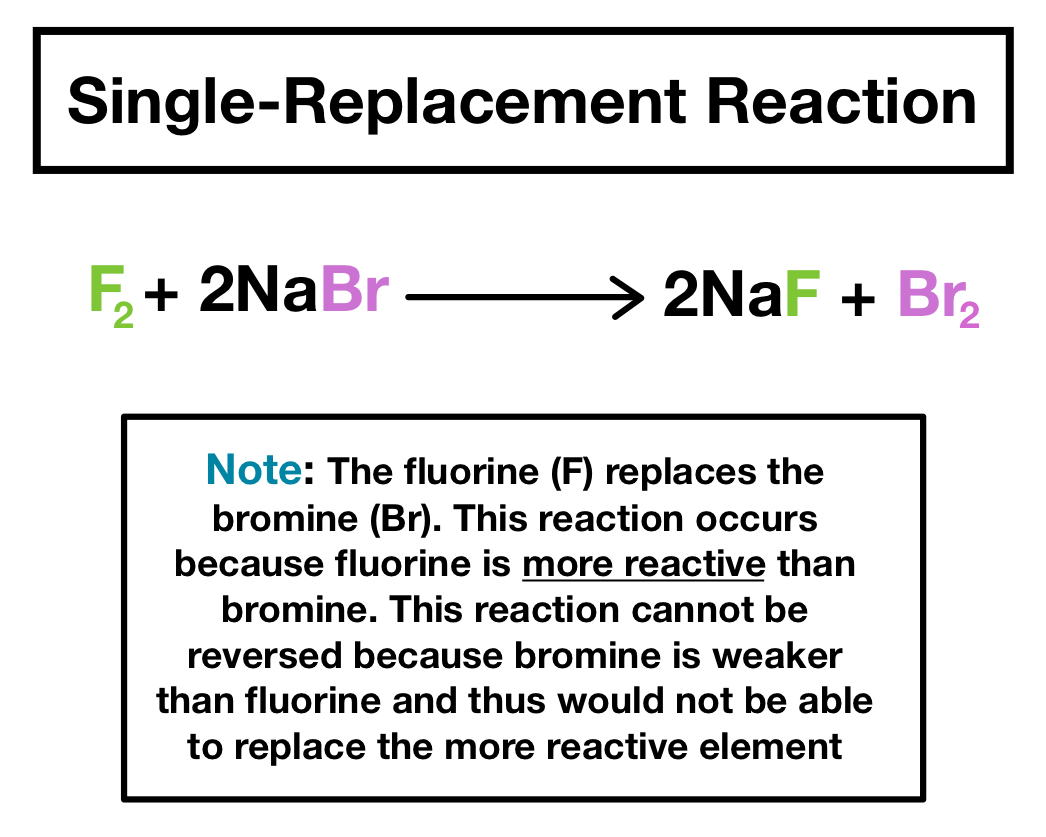
Single Replacement Reactions Definition Examples Expii

Single Replacement Single Displacement Reaction

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
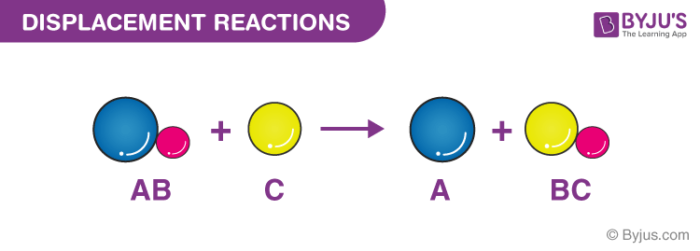
Displacement Reactions Definition Types Single Double Examples
0 comments
Post a Comment